How Clinical Trials Have Evolved Post COVID-19

The race to produce new drugs and vaccines for COVID-19, has demonstrated the importance of accelerating the process of drug discovery and approval. Studies show that introducing a drug to the marketplace can take 10 years on average. Thanks to COVID-19, the worldwide clinical trials landscape has advanced faster in the last six months than it has in the past 10 years.
In this article, we take a deeper look at the past, present, and future prospects of the clinical trial landscape, which continues to evolve as we move forward.
COVID-19-fuelled disruptions in clinical trials
With COVID-19 being declared a global pandemic on March 11, 2020, the life sciences industry saw the suspension or disruption of an estimated 80% of non-COVID-19 trials. The US Food and Drug Administration (FDA) also produced guidelines for ensuring patient safety during the COVID-19 pandemic in March 2020 (and revised it again in July).
Staff furloughs, social-distancing protocols, financial setbacks, and concerns over patient safety wreaked havoc on investigative site capabilities. Sponsors, CROs, and other drug development support organizations had to move to remote working environments. In many cases, trials that were halted were barred from registering new patients. Patients who were already involved mostly continued to receive treatment while organizations and researchers worked to alter how care was provided in response to the pandemic impact.
One of the main disruptions to worldwide clinical trial research during COVID-19 was physical separation to ensure patient and researcher protection. Numerous researchers were pulled away from clinical trials to work in emergency medical care, particularly in areas where the pandemic threatened to overwhelm intensive care services.
Clinician’s response
Owing to COVID-19, 23 out of 25 organizations reported some level of impact on ongoing worldwide clinical trials, as per a survey conducted by the Tufts Center for the Study of Drug Development, in 2020. The survey results further show that very few organizations reported minor or no disruptions. These organizations were routinely conducting early-stage trials, in which medical follow-ups to recognize possible risks could be easily performed via telehealth.
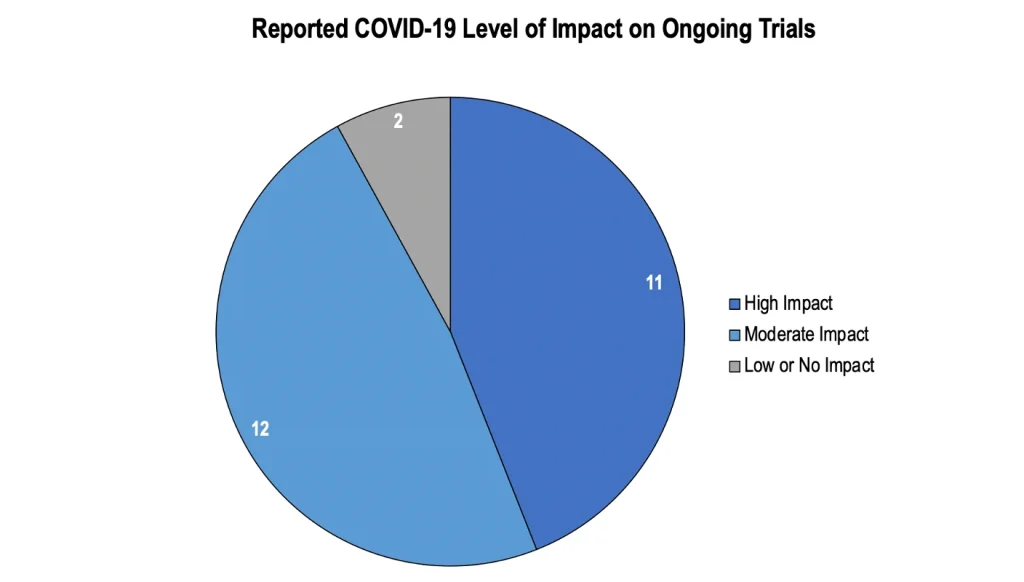
Source: Applied clinical trials
R&D organizations reported varying degrees of success in responding to COVID-related challenges. The organizations that self-reported “successful” COVID-19 responses frequently exhibited at least one of four key factors: organizational adaptability, previous investment in remote innovations, creation of COVID-19 task forces, and/or strong R&D skills in Asia-Pacific (APAC) territories. The formation of a COVID-19 task force was critical for larger firms to gear up and quickly modify worldwide clinical trial designs to include remote methodologies.
Adapting to virtual trials
As the pandemic gave way for an increased shift to virtual trials, it was time for the integration of remote technologies. For this, seasoned team members with backgrounds in operations, regulatory, and data processing were all needed. Since the beginning of 2020, the most commonly cited technologies have been telemedicine and eConsent.
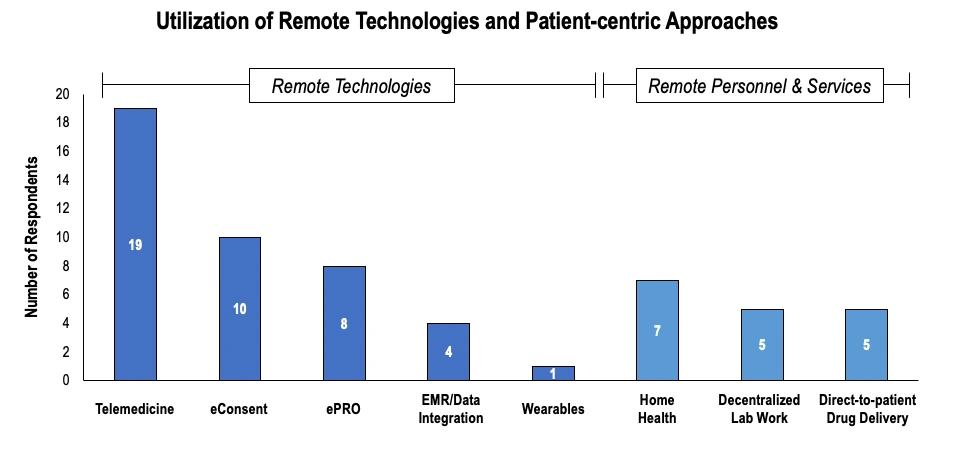
Source: Applied clinical trials
Ultimately, as virtual trials became popular, clinical operations moved away from drug distribution at the trial site and toward direct-to-patient delivery services, in which trial drugs are allocated and dispensed to patients in their homes, eliminating the need for high-risk patients to visit trial sites. Many in-person consultations for checkups and other facets of trials have since been replaced with teleconferencing offerings.
The acceleration of decentralized clinical trials
Virtual or decentralized clinical trials (DCT) are not new. They have been used, albeit sparsely, all over the world, for the past few years. However, with the advent of new remote technologies, clinical research organizations (CROs) and life science organizations were finally able to conduct decentralized, virtual clinical trials more easily. There was also a need for regulatory assistance and versatility for patients in ongoing trials, especially in restricted areas with reduced mobility, which further helped speed up this process.
“The pandemic has accelerated the shift to decentralized clinical trials. Now, we finally have the technology that is needed to facilitate this change, which did not exist 5-6 years ago. Whether it’s the high data transfer speeds or innovative and secure data collection devices,” says Dr. Jitesh Bhatt, VP, Clinical Product, Wellthy Therapeutics.
Different approaches to clinical trials in the current scenario
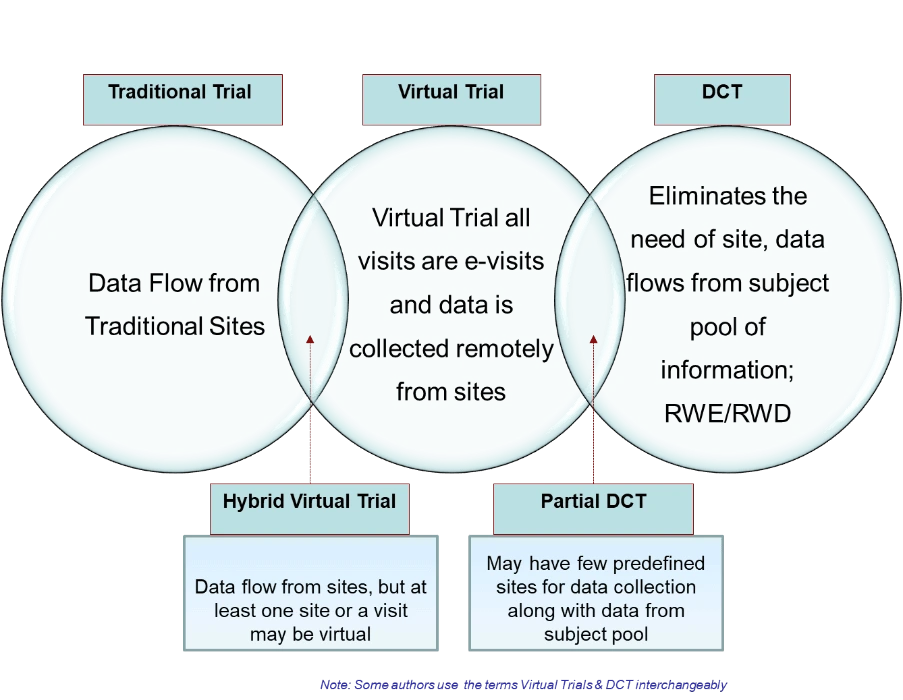
Source: HCL technologies
Industry experts are also determined to make this practice of administering decentralized trials the new standard. The Decentralized Trials & Research Alliance (DTRA) is working to bring together industry stakeholders with the common goal of making clinical trial engagement more accessible by expanding policies, applied research, and investing in technological innovations in decentralized clinical research.
Smart technology, in addition to innovative platforms like IQVIA Virtual Trials, is also assisting clinicians and pharmaceutical organizations in becoming used to conducting DCTs or hybrid trials. Some healthcare experts believe that devices like Swittons, which is a customizable enterprise platform, are completely transforming clinical trial procedures. Smartphones, wearables, artificial intelligence, machine learning, cloud computing, and blood collection devices are some of the other tools that enable DCTs.
Barriers to DCT adoption
However, there is still a way to go when it comes to making the DCT process smoother and error-free. Dr. Bhatt says, “One of the core challenges that DCT clinicians face is cross-functional alignment. Almost all DCTs these days use a technology known as electronic clinical outcome assessment (e-COA). This has made it very easy for CROs and sponsors to process the trial data faster, and also helps them in filing for regulatory approvals. However, e-COA vendors have a huge dependency on other third-party vendors, who take care of where the data is coming from, how it’s coming, and its compliance, and this is where gaps can exist.”
The solution, regardless of the nature of the gaps, lies in making eCOA platforms more efficient electronically, which again points us in the direction of using new technologies. Even when it comes to more practical issues, such as a decline in patient attitudes towards virtual trials, efforts are being made in terms of innovations, to mitigate such challenges. Trial researchers and principal investigators are also being trained to become more proficient in using the emerging modalities, and trial designs.
What does the future look like?
Clinical trials are an important tool in modern medicine, but COVID-19 has revealed several ways to enhance their design, operation, and documentation. The accelerated design and launch of COVID-19 clinical trials have shown that some facets and procedures of such trials could be changed, simplified, or revamped in ways that would favor patients, physicians, and all researchers. To achieve the best level of work in the future, it would be necessary to integrate those lessons into the research process.
Regulatory changes
To establish the role of virtual approaches and foster patient-centricity in future clinical trials, the industry, regulators, and investigating sites will need to take several steps. To encourage market acceptance, authorities must take a firm stand on the use of digital and remote technology in clinical trials. Currently, regulators’ lack of clarity about how remotely gathered information can be approved causes a degree of reluctance amongst life sciences companies to implement emerging technologies.
According to Rob Church, a pharmaceutical and biotechnology law expert, however, there is a clear indication that the FDA plans to increase the adoption of electronic tools in trials, moving forward. “Many of our clients, both on the pharma side as well as on the service industry side, are trying to come to terms with what standards the digital technologies and devices, whether they are wearables or apps, need to comply with, before they are used in studies. This is a real area of interest for the industry right now, but also something the FDA is paying attention to,” he added.
Technological innovations
On another front, technological advancements are also pushing clinical research into a new era. According to Dr. Bhatt, “technological shifts such as the use of Natural Language Processing (NLP) through conversational AI chatbots, can help maintain the physician-patient relationship and also improve patient engagement, from a remote perspective.” He also mentioned devices such as image-based AI solutions and IoMT networks that will upscale virtual trials.
How different digital technologies are affecting the future of clinical trials
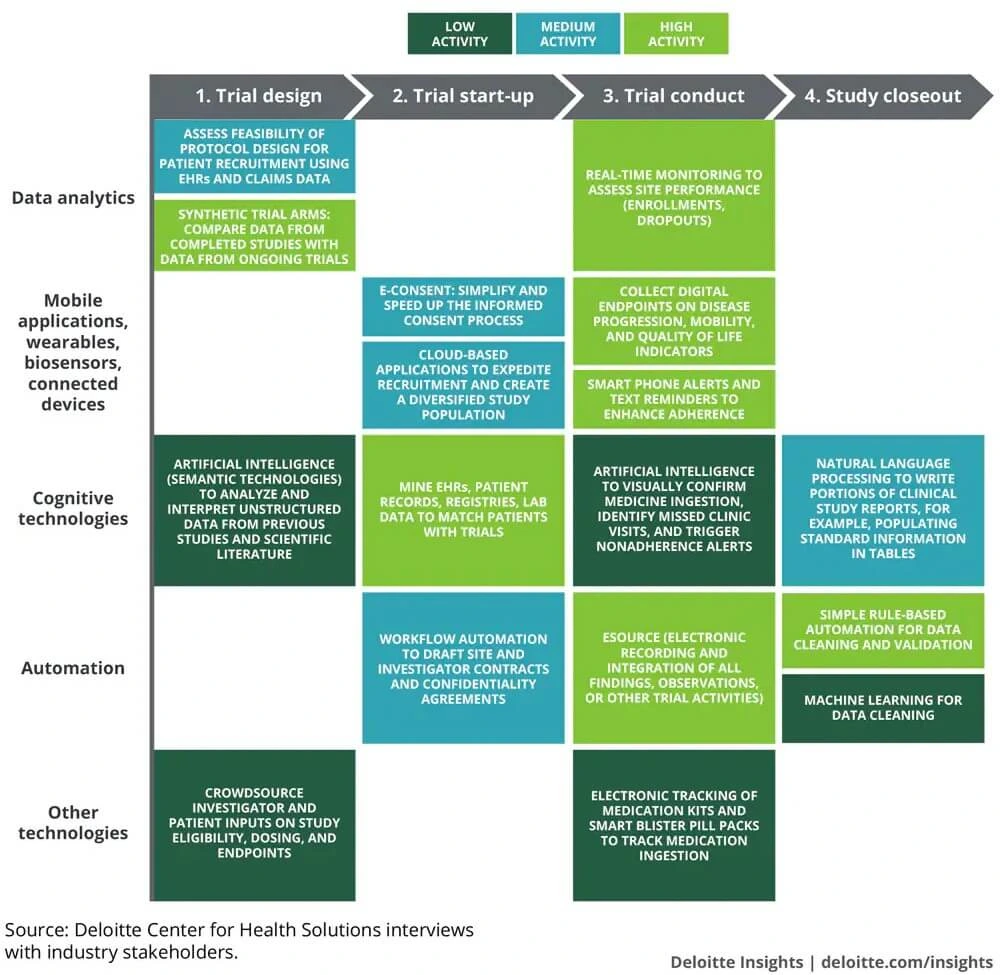
Source: Deloitte
Ultimately, systems that enable DCTs are working to clear the COVID-19 pandemic’s backlog in the therapy pipelines, and such innovations will expand on this to do much more. The possible scenarios for improving the quality of care and patient outcomes become more interesting as technology firms, researchers, scientists, and the government collaborate to optimize and enhance the clinical trial procedures.
Netscribes offers healthcare market research services to companies around the world, assisting them in gaining holistic insights into therapy area pipelines, in-licensing potential, trial tracking, treatment flows, and guidelines, etc. Contact us, to know more.
Based on inputs by Dr. Jitesh Bhatt, VP, Clinical Product, Wellthy Therapeutics.